Synthesis and X-ray absorption spectroscopy of potassium transition metal fluoride nanocrystals†
Abstract
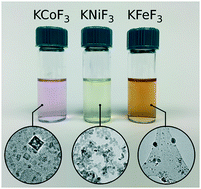
Nanocrystals of KMF3 (M = Mn–Ni) and K3MF6 (M = V, Fe) were synthesized via non-aqueous routes based on colloidal chemistry. The effect of a variety of parameters on the purity, size and quality of the nanocrystals was evaluated. Fluorides formed from mixtures of commercially available potassium trifluoroacetate and transition metal alkoxides, as opposed to existing methods based solely on trifluoroacetate precursors. Particles of KMF3 in the range of several to tens of nanometers, were achieved. It was found that methodologies based on hot injection of precursors often led to crystal sizes an order of magnitude smaller than those obtained from co-thermolysis as well as higher purity material. X-ray absorption spectroscopy revealed that M–F bond hybridization increased with transition metal d-electron count. On further probing of the Fe K and L edges, making use of different probing depths, KFeF3 nanocrystals were found to have FeII in the bulk, while the surface was rich in FeIII, but not as the product of oxidation. The developed protocols and lessons learned could be leveraged to rapidly devise synthetic methods for other alkali transition metal fluorides of interest.


 Please wait while we load your content...
Please wait while we load your content...