One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride†
Abstract
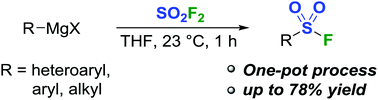
Herein, we report a new method for the one-pot syntheses of sulfonyl fluorides. Addition of an alkyl, aryl, or heteroaryl Grignard to a solution of sulfuryl fluoride at ambient temperature affords the desired sulfonyl fluorides in 18–78% yield. Furthermore, this method is applicable for in situ sequential reactions, whereby the Grignard reagent can be converted to the corresponding diarylsulfone, sulfonate ester, or sulfonamide in a one-pot process.


 Please wait while we load your content...
Please wait while we load your content...