Visible-light unmasking of heterocyclic quinone methide radicals from alkoxyamines†
Abstract
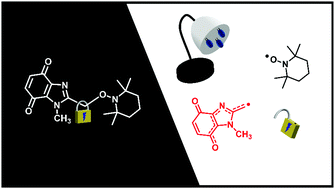
In nature, the unmasking of heterocyclic quinones to form stabilized quinone methide radicals is achieved using reductases (bioreduction). Herein, an alternative controllable room-temperature, visible-light activated protocol using alkoxyamines and bis-alkoxyamines is provided. Selective synthetic modification of the bis-alkoxyamine, allowed chromophore deactivation to give one labile alkoxyamine moiety.


 Please wait while we load your content...
Please wait while we load your content...