Catalytic asymmetric cycloaddition of unsymmetrical EWG-activated alkenes to fully substituted pyrrolidines bearing three different carbonyl groups†
Abstract
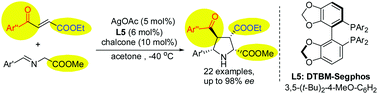
A unique 1,3-dipolar [3+2] cycloaddition of alkyl 4-oxo-4-arylbut-2-enoates bearing two different electron-withdrawing groups was completed by using the silver/(R)-DTBM-Segphos catalyst system, which gives the corresponding fully substituted pyrrolidines with four stereogenic centers in good yields and with excellent enantioselectivities (up to 98% ee).


 Please wait while we load your content...
Please wait while we load your content...