Photocatalytic hydroacylation of trifluoromethyl alkenes†
Abstract
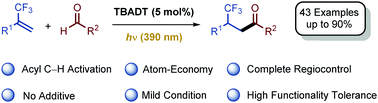
Hydrofunctionalization of trifluoromethyl alkenes is highly challenging, because the nucleophilic addition is oftentimes followed by β-F elimination in this case. By the use of tetrabutylammonium decatungstate (TBADT) as a hydrogen-atom-transfer (HAT) photocatalyst for acyl C–H activation, we successfully avoid the β-F elimination in the hydroacylation of trifluoromethyl alkenes with aldehydes. This light (390 nm) promoted reaction provides a facile and efficient access to various β-CF3 ketones in complete regiocontrol with high functionality tolerance and 100% atom economy.


 Please wait while we load your content...
Please wait while we load your content...