Stereoselective three-component cascade synthesis of α-substituted 2,4-dienamides from gem-difluorochloro ethanes†
Abstract
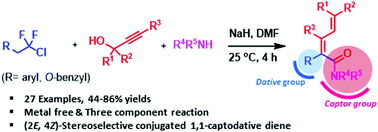
Herein, we describe a new transition metal-free Claisen rearrangement for the synthesis of α-substituted 2,4-dienamides. The one-pot, stereoselective three-component cascade reaction between a series of propargyl alcohols, amines, and gem-difluorochloro ethane derivatives afforded various polysubstituted 2,4-dienamides in good yields. This synthetic method for 1,1-captodative dienes, α-substituted 2,4-dienamides, can be utilized for preparing pharmaceutical analogues containing an indolin-2-one or lactone moiety.


 Please wait while we load your content...
Please wait while we load your content...