Tryptophan–glucosamine conjugates modulate tau-derived PHF6 aggregation at low concentrations†
Abstract
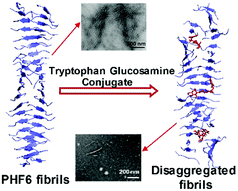
Glycosylation of amyloidogenic proteins enhances their solubility and reduces propensity for aggregation. We therefore, prepared tryptophan–glucosamine conjugates to modulate aggregation of tau-derived PHF6-peptide. Combined in vitro and in silico approaches indicated that these conjugates inhibited oligomerization and fibril formation of PHF6 and disrupted its preformed fibrils at very low concentration. These effects mainly arise from the glucopyranoside moiety.


 Please wait while we load your content...
Please wait while we load your content...