Ultramacrocyclization via selective catenation in water†
Abstract
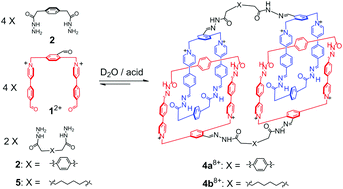
A set of two cyclic [2]catenane dimers were self-assembled in aqueous media via selective hydrazone condensation, by means of either one-pot or post-functionalization procedures. Considering that each catenane is composed of two thirty-six member rings, each of these two cyclic [2]catenane dimers can be considered as a ultra-large macrocycle that contains more than fifty atoms in the ring framework. This cyclization methodology relying on catenation thus represents a novel approach for accomplishing ultramacrocyclization in pure water.


 Please wait while we load your content...
Please wait while we load your content...