Sodium chromium hexacyanoferrate as a potential cathode material for aqueous sodium-ion batteries†
Abstract
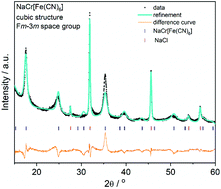
Sodium chromium hexacyanoferrate (NaCrHCF) is obtained here using a facile co-precipitation method at room temperature. The powder was investigated in terms of potential use as a cathode material for aqueous sodium-ion batteries under neutral conditions. The highest achieved discharge capacity of NaCrHCF was around ∼64 mA h g−1 at C/3 current rate.


 Please wait while we load your content...
Please wait while we load your content...