Divergent gold-catalysed reactions of cyclopropenylmethyl sulfonamides with tethered heteroaromatics†
Abstract
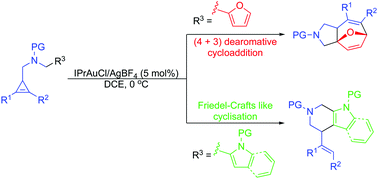
Cyclopropenylmethyl sulfonamides with tethered heteroaromatics have been demonstrated to undergo divergent gold-catalysed cyclisation reactions. A formal dearomative (4+3) cycloaddition takes place with furan-tethered substrates, yielding densely functionalised 5,7-fused heterocycles related to the bioactive curcusone natural products. Indole-tethered substrates display divergent reactivity giving biologically important tetrahydro-β-carbolines via a Friedel–Crafts mechanism.


 Please wait while we load your content...
Please wait while we load your content...