Perfluorophenylboronic acid-catalyzed direct α-stereoselective synthesis of 2-deoxygalactosides from deactivated peracetylated d-galactal†
Abstract
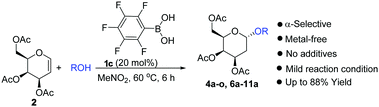
Perfluorophenylboronic acid 1c catalyzes the direct stereoselective addition of alcohol nucleophiles to deactivated peracetylated D-galactal to give 2-deoxygalactosides in 55–88% yield with complete α-selectivity. The unprecedented results reported here also enable the synthesis of disaccharides containing the 2-deoxygalactose moiety directly from the deactivated peracetylated D-galactal. This convenient and metal-free glycosylation method works well with a wide range of alcohol nucleophiles as acceptors and tolerates a range of functional groups without the formation of the Ferrier byproduct and without the need for a large excess of nucleophiles or additives. The method is potentially useful for the synthesis of a variety of α-2-deoxygalactosides.


 Please wait while we load your content...
Please wait while we load your content...