Endergonic addition of N-methylamines to aromatic ketones driven by photochemical offset of the entropic cost†
Abstract
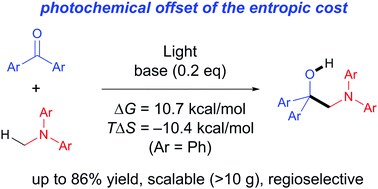
Intermolecular addition reactions are generally accompanied by an entropic penalty due to the decrease of molecular numbers during the reaction, which sometimes makes the reaction endergonic. Here we demonstrate that such an endergonic reaction can be promoted with light-energy as a driving force; N-methylamines were added to aromatic ketones to produce aminoalcohols under UV-light irradiation. The reaction represents an obvious example showing that the photochemical approach is effective to offset such an entropic cost, and thereby to drive thermodynamically uphill addition reactions. Moreover the present reactions are highly expedient from the synthetic view point, being transition-metal-catalyst-free, scalable, highly atom economical, and regioselective. The product amines can be converted in one step to functional multi-arylated enamines, which are potentially valuable compounds in electronic materials.


 Please wait while we load your content...
Please wait while we load your content...