Access to C-aryl/alkenylglycosides by directed Pd-catalyzed C–H functionalisation of the anomeric position in glycal-type substrates†
Abstract
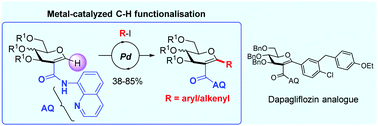
Directed palladium-catalyzed C–H functionalisation of C2-amido glycals onto the anomeric position is described as a novel route to C-aryl/alkenylglycosides. An aminoquinoline-type directing group was used to successfully introduce diverse (hetero)aryl and alkenyl groups at position 1 of the sugar (20 examples). Its application to the synthesis of a dapagliflozin analogue is presented.


 Please wait while we load your content...
Please wait while we load your content...