C–H and N–H bond annulation of aryl amides with unactivated olefins by merging cobalt(iii) and photoredox catalysis†
Abstract
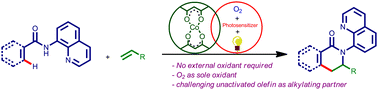
A mild, environment-friendly protocol has been developed to carry out the [4+2] annulation of aryl amides with unactivated olefins. A range of sterically and electronically diverse aryl amides and unactivated olefins were successfully employed under the developed conditions to get a variety of dihydroisoquinolinones in good-to-excellent yields.


 Please wait while we load your content...
Please wait while we load your content...