A newly designed heterodiene and its application to construct six-membered heterocycles containing an N–O bond†
Abstract
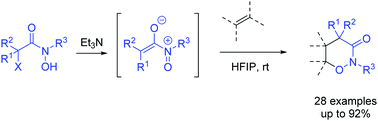
A new type of zwitterionic nitrosoalkene generated in situ from dehydrohalogenation of α-halo-N-alkylhydroxamic acids was designed. [4+2] cycloadditions of this heterodiene to olefins provide a facile route to construct six-membered heterocycles containing an N–O bond and a new protocol for 1,2-syn carbohydroxylate alkenes with a hydroxyl and acetamide group. DFT calculations support a concerted cycloaddition pathway and the solvent HFIP can stabilize the transition state through H-bonding interaction.


 Please wait while we load your content...
Please wait while we load your content...