A practical copper-catalyzed approach to β-lactams via radical carboamination of alkenyl carbonyl compounds†
Abstract
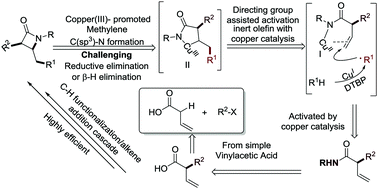
Functionalized β-lactams are highly important motifs in synthetic chemistry. We report an efficient and novel approach to the synthesis of β-lactams via a copper(I)-catalyzed cascade process involving C(benzyl)–H radical abstraction, intermolecular alkene addition, and intramolecular amination reaction. Variously substituted alkenes were synthesized from vinylacetic acid, leading to the corresponding β-lactams in moderate to good yields. Preliminary studies indicate that the reaction undergoes a free radical mechanism via a Cu(I)/Cu(II)/Cu(III) catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...