Intramolecular benzoallene–alkyne cycloaddition initiated by site-selective SN2′ reaction of epoxytetracene en route to π-extended pyracylene†
Abstract
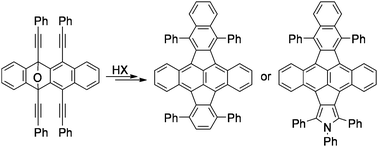
A hydrogen halide promoted cascade reaction of epoxytetracene to afford halo-benzoindenotetracene including a benzoallene intermediate was developed. The remaining two alkynyl groups in benzoindenotetracene were further reacted with norbornadiene or arylamine through transition metal-catalyzed cyclization to give π-extended pyracylene derivatives.


 Please wait while we load your content...
Please wait while we load your content...