Tandem double hydrophosphination of α,β,γ,δ-unsaturated-1,3-indandiones: diphosphine synthesis, mechanistic investigations and coordination chemistry†
Abstract
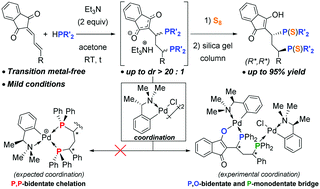
A metal-free tandem double hydrophosphination of extended conjugated indandiones has been established. Mechanistic investigations confirmed the consecutive manner of the nucleophilic addition reaction. Complexation of the generated keto-diphosphine resulted in the formation of an unexpected tridentate bridging ligand with an anionic P,O-bidentate and a neutral P-monodentate coordination mode on two palladium units. In the presence of an external chiral auxiliary, the coordinated diphosphines could be separated into their enantiomeric forms.


 Please wait while we load your content...
Please wait while we load your content...