Nickel/copper-cocatalyzed decarbonylative silylation of acyl fluorides†
Abstract
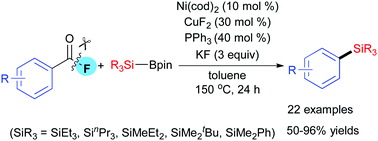
Ni/Cu-cocatalyzed decarbonylative silylation of acyl fluorides with silylboranes has been developed to afford various arylsilanes with high efficiency and good functional-group compatibility via carbon–fluorine bond cleavage and carbon–silicon bond formation. Such transformation can not only extend the functionalization type of acyl fluorides but complement the synthetic route for arylsilanes.


 Please wait while we load your content...
Please wait while we load your content...