Photoredox asymmetric catalytic enantioconvergent substitution of 3-chlorooxindoles†
Abstract
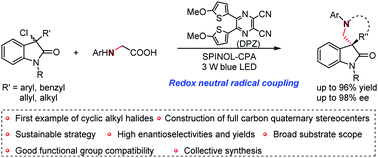
An enantioconvergent substitution of 3-substituted 3-chlorooxindoles with N-aryl glycines under visible light irradiation is reported. A transition-metal-free cooperative catalysis platform with a dicyanopyrazine-derived chromophore (DPZ) as a photoredox catalyst and a chiral Brønsted acid catalyst is effective for these transformations, which involve a single-electron transfer redox step and an enantioselective radical coupling. A variety of valuable chiral 3-aminomethylene-3-substituted oxindoles can be directly synthesized with high yields and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...