Visible-light-mediated defluorinative cross-coupling of gem-difluoroalkenes with thiols†
Abstract
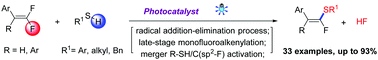
Here we report a visible-light-mediated monofluoroalkenylation through defluorinative cross-coupling of gem-difluoroalkenes with aryl, benzyl, and alkyl thiols. This novel strategy provides facile and efficient access to tri/tetra-substituted monofluoroalkenes under mild reaction conditions with good functional group tolerance. Late-stage modification of natural products indicated the synthetic potential of this S–H monofluoroalkenylation process.


 Please wait while we load your content...
Please wait while we load your content...