Cyclic (aryl)(amido)carbenes: pushing the π-acidity of amidocarbenes through benzannulation†
Abstract
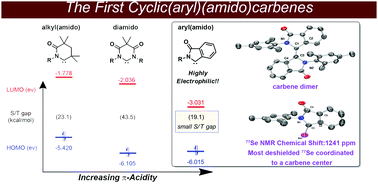
Cyclic(aryl)(amido)carbenes were synthesized, and studied via a combination of experimental and computational approaches. These carbenes undergo dimerization when isolation is attempted, however, are trapped with sulfur, selenium, and [Ir(cod)Cl]. The π-acidity, measured using 77Se NMR, revealed that these are the most electrophilic singlet carbenes reported to date whereas the TEP measured demonstrated that these carbenes are poor σ donors.


 Please wait while we load your content...
Please wait while we load your content...