Electronic and spin delocalization in a switchable trinuclear triphenylene trisemiquinone bridged Ni3 complex†
Abstract
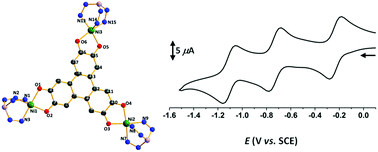
A trinuclear triphenylene trisemiquinone complex containing paramagnetic NiII is obtained under ambient conditions from the reaction of deprotonated tricatecholate hexahydroxytriphenylene (H6HHTP) with NiII capped with a trispyrazolyl borate tridentate ligand. The magnetic and EPR data are consistent with delocalization of the electronic spin over the three NiII species. The two-electron reduced complex shows an EPR spectrum corresponding to a S = 1/2 species due to a large antiferromagnetic coupling between the radical and only one of the NiII ions highlighting the localization of the electronic spin. No EPR signal is observed for the one- and three-electron reduced species consistent with the closed shell of the bridging ligand.


 Please wait while we load your content...
Please wait while we load your content...