Pd-Catalyzed decarboxylative cross-coupling reactions of epoxides with α,β-unsaturated carboxylic acids†
Abstract
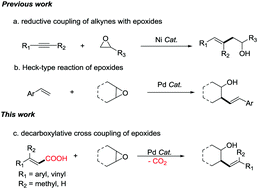
A Pd-catalyzed decarboxylative cross-coupling of α,β-unsaturated carboxylic acids with cyclic and acyclic epoxides has been developed. Both β-monosubstituted and β-disubstituted unsaturated carboxylic acids, as well as conjugated diene unsaturated carboxylic acids are suitable reaction substrates. Substituted homoallylic alcohols were obtained in moderate to good yields. The product was obtained as a mixture of diastereomers favoring the anti diastereomer of the cyclic epoxides. This work provides a method for the modification of complex organic molecules containing α,β-unsaturated carboxylic acids.
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...