Metal-free defluorinative arylation of trifluoromethyl alkenes via photoredox catalysis†
Abstract
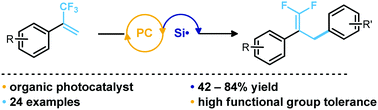
Literature methods to access gem-difluoroalkenes are largely limited to harsh, organometallic-based methods, and known photoredox-mediated processes are not amenable to aryl radical addition to trifluoromethyl alkenes. A metal-free, functional group-tolerant method for the preparation of benzylic gem-difluoroalkenes is described. Halogen atom abstraction from (hetero)aryl halides generates aryl radicals that undergo a defluorinative arylation of α-trifluoromethyl alkenes, tolerating electronically disparate aryl radicals and α-trifluoromethyl alkenes.


 Please wait while we load your content...
Please wait while we load your content...