Rational redox tuning of transition metal sites: learning from superoxide reductase†
Abstract
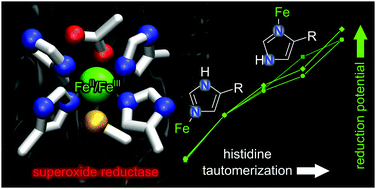
Using superoxide reductase as a model system, a computational approach reveals how histidine tautomerism tunes the redox properties of metalloenzymes to enable their catalytic function. Inspired by these experimentally inaccessible insights, non-canonical histidine congeners are introduced as new versatile tools for the rational engineering of biological transition metal sites.


 Please wait while we load your content...
Please wait while we load your content...