Intramolecular nitration–aminocarbonylation of unactivated olefins: metal-free synthesis of γ-lactams†
Abstract
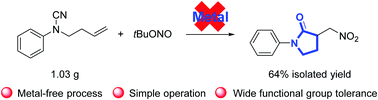
Disclosed herein is a novel, metal-free synthesis of γ-lactams through the radical-mediated nitration–aminocarbonylation of unactivated olefins. The reaction is initiated by a nitro radical generated from the homolysis of tert-butyl nitrite. The intramolecular cyanamide serves as the aminocarbonylating reagent. This protocol offers an environment-benign method to produce the synthetically valuable nitromethyl substituted γ-lactams.


 Please wait while we load your content...
Please wait while we load your content...