Kinetically controlled asymmetric synthesis of silicon-stereogenic methoxy silanes using a planar chiral ferrocene backbone†
Abstract
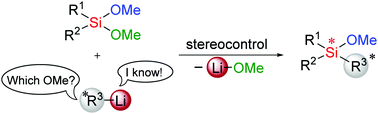
We present a mechanistic proposal for the desymmetrisation of dimethoxy silanes with alkyllithiums. The stereochemical pathway is highly defined by the coordination of the metal lithium, which suppresses the reversible interconversion of the various pentavalent intermediates. The predicted kinetic control of the stereoinduction is verified by the experiments using a planar chiral ferrocene backbone.


 Please wait while we load your content...
Please wait while we load your content...