Pd-catalysed selective C(sp3)–H arylation and acetoxylation of alcohols†
Abstract
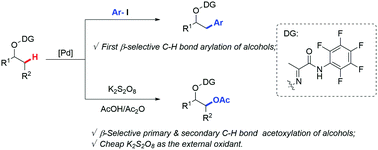
A palladium catalyzed selective C(sp3)–H arylation and acetoxylation of alcohols using a practical bidentate auxiliary were developed. Masked alcohols were selectively arylated at the β-position with diverse aryl iodides for the first time. Moreover, an efficient and site-selective acetoxylation of various primary methyl, methylene, and benzylic C(sp3)–H bonds was performed by using cheap K2S2O8 as the external oxidant.


 Please wait while we load your content...
Please wait while we load your content...