PdCl2(CH3CN)2-catalyzed regioselective C–H olefinations of 2-amino biaryls with vinylsilanes as unactivated alkenes†
Abstract
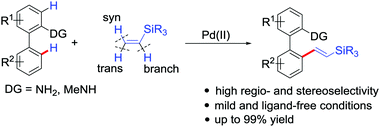
The first example of palladium-catalyzed chelation-assisted C–H olefination of 2-amino biaryls using readily available vinylsilanes as unactivated olefinating reagents has been developed, affording valuable arylated vinylsilane compounds in moderate to excellent yields and with exclusive (E)-selectivities.


 Please wait while we load your content...
Please wait while we load your content...