4-Hydroxymethyl-substituted oxazolidinone synthesis by tetraarylphosphonium salt-catalyzed reactions of glycidols with isocyanates†
Abstract
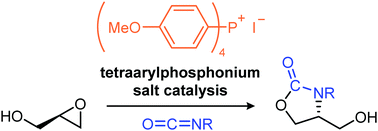
Preparation of 4-hydroxymethyl-substituted oxazolidinones including optically active ones, in which tetraarylphosphonium salts catalyze the reaction of glycidol with isocyanates effectively, is described. The neutral catalysis facilitates glycidol addition to isocyanates followed by intramolecular cyclization. Mechanistic studies suggest that hydrogen-bonding interactions with the catalyst play an important role in the reaction progress.


 Please wait while we load your content...
Please wait while we load your content...