Regioselective three-component synthesis of 1,2-diarylindoles from cyclohexanones, α-hydroxyketones and anilines under transition-metal-free conditions†
Abstract
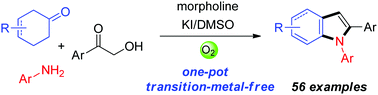
A facile method for the one-pot synthesis of 1,2-diarylindoles under transition-metal-free conditions is described. Cyclohexanones were used as the aryl sources via the dehydrogenative aromatization process. One C–C and two C–N bonds were selectively formed via a domino reaction. This protocol provides a convenient approach for the construction of valuable bioactive 1,2-diarylindoles from readily available cyclohexanones, α-hydroxyketones and anilines.

 Please wait while we load your content...
Please wait while we load your content...