Diastereoselective synthesis of cyclopropanes bearing trifluoromethyl-substituted all-carbon quaternary centers from 2-trifluoromethyl-1,3-enynes beyond fluorine elimination†
Abstract
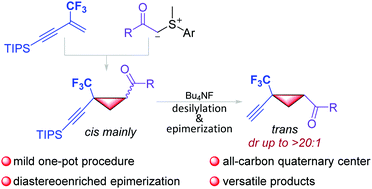
Herein, a one-pot, two-step procedure for the diastereoselective synthesis of cyclopropanes bearing trifluoromethyl-substituted all-carbon quaternary centers has been described. Trifluoromethyl-activated 1,3-enynes undergo cyclopropanation reactions with sulfur ylides under mild reaction conditions without fluoride elimination, which affords the cis-isomer mainly. Interestingly, a sequential TBAF-mediated deprotection of the triisopropylsilyl group results in a diastereoenriched epimerization which gives rise to the trans-cyclopropanes as the sole isomers.


 Please wait while we load your content...
Please wait while we load your content...