Synthesis of per(5-N-carboxamide-5-dehydroxylmethyl)-β-cyclodextrins and their selective recognition ability utilizing multiple hydrogen bonds†
Abstract
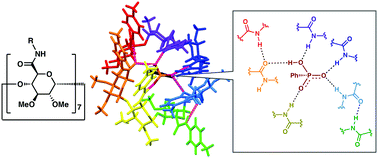
Per(5-N-carboxamide-5-dehydroxylmethyl)-β-cyclodextrin derivatives with seven equivalent amide groups directly attached to each pyranose ring were synthesized. The amide cyclodextrins show unique recognition properties toward hydrogen phosphonate anions. An X-ray crystallographic analysis revealed its recognition mode in which unsymmetrically arranged amide groups play distinctive roles both as a hydrogen bond donor and acceptor.


 Please wait while we load your content...
Please wait while we load your content...