Silylene induced cooperative B–H bond activation and unprecedented aldehyde C–H bond splitting with amidinate ring expansion†
Abstract
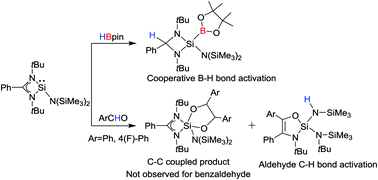
The addition of HBpin to PhC(NtBu)2SiN(SiMe3)2 (1) results in the cleavage of the B–H bond in a cooperative fashion across the Si and amidinate-C sites. The reaction of 1 with benzaldehyde led to C–H bond activation with amidinate ring expansion leading to a five-membered heterocycle. In case of 4-fluorobenzaldehyde, a C–C bond coupling takes place leading to a dioxasilolane derivative as the major product.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...