A rhodium-catalysed Sonogashira-type coupling exploiting C–S functionalisation: orthogonality with palladium-catalysed variants†
Abstract
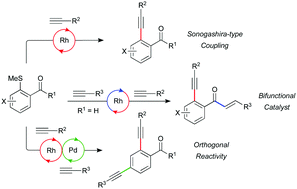
This report concerns the development of an efficient Sonogashira-type coupling of arylmethylsulfides and terminal alkynes to generate aryl alkyne motifs. Orthogonal reactivity between traditional Pd catalysts, and the Rh catalysts employed, results in the ability to selectively activate either the C–S bond or C–X bond through catalyst choice. The Rh–bisphosphine catalyst has further been shown to be able to effect a hydroacylation-Sonogashira tandem sequence, and in combination with further onward reactions has been used in the synthesis of heterocycles and polycyclic systems.


 Please wait while we load your content...
Please wait while we load your content...