A chemoselective reaction between protein N-homocysteinylation and azides catalyzed by heme(ii)†
Abstract
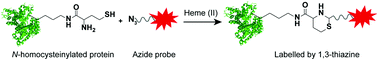
Herein, we present a serendipitously discovered chemoselective labelling of protein N-homocysteinylation with bioorthogonal azide probes. The reaction proceeds rapidly under alkaline and heating conditions. Our experiments suggest that azides can be converted to aldehydes in situ catalyzed by heme(II), followed by a condensation with protein N-homocysteinylation to afford stable 1,3-thiazines.


 Please wait while we load your content...
Please wait while we load your content...