Concentrated-acid triggered superfast generation of porous amorphous cobalt oxide toward efficient water oxidation catalysis in alkaline solution†
Abstract
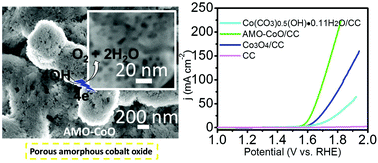
Amorphous transition-metal (hydr)oxides have proven to be the most promising oxygen evolution reaction (OER) electrocatalysts. Here, a facile and novel strategy for the generation of porous amorphous cobalt oxide through one-step treatment of the cobalt carbonate hydroxide precursor with concentrated sulfuric acid has been reported. Benefiting from the optimized amorphous structure, the prepared catalyst exhibits an excellent water-oxidation activity in an alkaline electrolyte with the demand of 50 mV less overpotential at 10 mA cm−2 compared to that of highly-crystallized Co3O4. Compared with the 400 °C annealing process for Co3O4, the ultrafast acid treatment process within 10 seconds at room-temperature for achieving such amorphous cobalt oxide demonstrates tremendous convenience. More impressively, after continuous testing for 60 h at an overpotential of 370 mV, it still maintains a porous configuration as well as ∼92% of the original current density. Our findings not only present a high-efficiency OER electrocatalyst, but also provide a methodology with broad applicability toward the systematic study of other non-crystal oxides for green energy applications.


 Please wait while we load your content...
Please wait while we load your content...