Organophosphine-free copper-catalyzed isothiocyanation of amines with sodium bromodifluoroacetate and sulfur†
Abstract
A copper-catalyzed isothiocyanation of amines with sodium bromodifluoroacetate and sulfur in the absence of organophosphine has been established. This approach represents a simple and efficient one-pot synthesis of isothiocyanates, and features excellent functional group tolerance and the use of a cheap, safe and odorless sulfur source. Moreover, this process could directly provide isothiocyanate analogous bioactive molecules, thiocarbonyl-containing pesticides and facile construction of benzoxazole and benzimidazole frames.
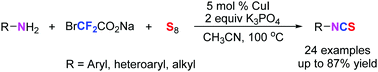


 Please wait while we load your content...
Please wait while we load your content...