Triplet-sensitised di-π-methane rearrangement of N-substituted 2-azabarrelenones†
Abstract
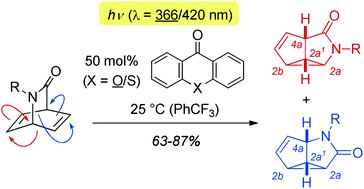
When irradiated at λ = 366 nm or at λ = 420 nm in the presence of an appropriate sensitiser the title compounds underwent a di-π-methane rearrangement which led to the formation of tricyclic azasemibullvalenones (2a,2a1,2b,4a-tetrahydroazacyclopropa[cd]pentalenones) in yields of 63–87%.


 Please wait while we load your content...
Please wait while we load your content...