Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering†
Abstract
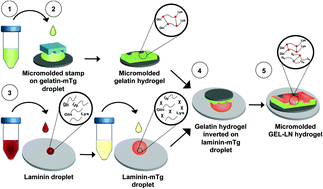
We report a water-soluble and non-toxic method to incorporate additional extracellular matrix proteins into gelatin hydrogels, while obviating the use of chemical crosslinkers such as glutaraldehyde. Gelatin hydrogels were fabricated using a range of gelatin concentrations (4%–10%) that corresponded to elastic moduli of approximately 1 kPa–25 kPa, respectively, a substrate stiffness relevant for multiple cell types. Microbial transglutaminase was then used to enzymatically crosslink a layer of laminin on top of gelatin hydrogels, resulting in 2-component gelatin–laminin hydrogels. Human induced pluripotent stem cell derived spinal spheroids readily adhered and rapidly extended axons on GEL-LN hydrogels. Axons displayed a more mature morphology and superior electrophysiological properties on GEL-LN hydrogels compared to the controls. Schwann cells on GEL-LN hydrogels adhered and proliferated normally, displayed a healthy morphology, and maintained the expression of Schwann cell specific markers. Lastly, skeletal muscle cells on GEL-LN hydrogels achieved long-term culture for up to 28 days without delamination, while expressing higher levels of terminal genes including myosin heavy chain, MyoD, MuSK, and M-cadherin suggesting enhanced maturation potential and myotube formation compared to the controls. Future studies will employ the superior culture outcomes of this hybrid substrate for engineering functional neuromuscular junctions and related organ on a chip applications.


 Please wait while we load your content...
Please wait while we load your content...