Tuning the matrix metalloproteinase-1 degradability of peptide amphiphile nanofibers through supramolecular engineering†
Abstract
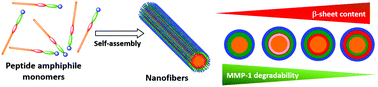
Matrix metalloproteinases (MMPs) are a family of endopeptidases capable of degrading extracellular matrix (ECM) components. They are known to play crucial roles during the ECM turnover in both physiological and pathological processes. As such, their activities are utilized as biological stimuli to engineer MMP-responsive peptide-based biomaterials such as self-assembled peptide amphiphiles (PAs). Although previous studies have unveiled the role of PAs secondary structure on the mechanical and biological properties of their self-assembled nanostructures, the effect on the degradability of their assemblies by MMP-1 has not been reported. Herein, a series of PAs are designed and synthesized, all comprising the same MMP-1 cleavable domain but with variable structural segments, to decipher the role of PA's secondary structure on the MMP-1 degradability of their assemblies. This study reveals a correlation between the MMP-1 degradation efficiency and the β-sheet content of the self-assembled PA nanofibers, with the MMP-1 cleavability being significantly reduced in the PA nanofibers with stronger β-sheet characteristics. These results shed light on the role of supramolecular cohesion in PA assemblies on their hydrolysis by MMP-1 and open up the possibility to control the degradation rate of PA-based nanostructures by MMP-1 through tweaking their molecular sequences.


 Please wait while we load your content...
Please wait while we load your content...