A self-assembly and higher order structure forming triple helical protein as a novel biomaterial for cell proliferation†
Abstract
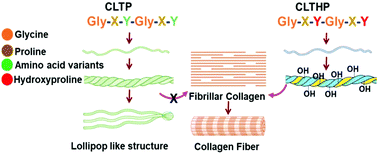
Collagen plays a critical role in the structural design of the extracellular matrix (ECM) and cell signaling in mammals, which makes it one of the most promising biomaterials with versatile applications. However, there is considerable concern regarding the purity and predictability of the product performance. At present, it is mainly derived as a mixture of collagen (different types) from animal tissues, where the selective enrichment of a particular type of collagen is generally difficult and expensive. Collagen derived from bovine sources poses the risk of transmitting diseases and can cause adverse immunologic and inflammatory responses. Hence, recombinant collagen can be a good alternative. Nevertheless, the necessity of post-translational hydroxyproline (Hyp) modification limits large-scale recombinant collagen production. Here, we recombinantly expressed the collagen-like protein (CLTP) and genetically introduced the Hyp in the CLTP to form a higher order self-assembled fibril structure, similar to human collagen. During the current study, it was observed that the Hyp incorporated CLTP protein (CLTHP) formed a stable triple helical polyproline-II like structure and self-assembled to form fibrils at neutral pH, which had an initial lag phase followed by a growth phase similar to animal collagen. In contrast, the higher order fibrillar assembly was missing in the nonhydroxylated CLTP. This study demonstrated that CLTHP self-association is based on the common underlying lateral interactions between triple helical structured proteins, where the hydroxyproline forms the significantly stable hydration network. Hence, this work will be the first fundamental empirical research for flexible modifications of recombinant collagen for structural analysis and biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...