A quadruple-labeling luminescence strategy for multiplexed immunoassay of 51 drugs in milk with an automated pretreatment system†
Abstract
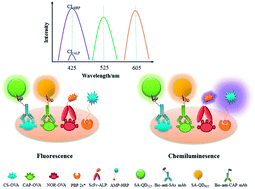
In this study, a novel combination of quadruple-labeling luminescence strategy multiplexed immunoassay (QLL-MIA) and an automated pretreatment system for simultaneous extraction and determination of 20 fluoroquinolones (FQs), 15 β-lactams, 15 sulfonamides (SAs), and chloramphenicol (CAP) in milk was developed. The strategy integrated 2 fluorescence probes, and 2 chemiluminescence probes to achieve signal separation, and a new combination of a nucleic acid extraction system and immunomagnetic beads (IMBs) as the automated pretreatment system to achieve simultaneous determination of 51 drugs. Taking advantage of automated pretreatment system for concentrating milk sample with 5 times and saving the extraction time, the limits of detection (LODs) for FQs, β-lactams, SAs, and CAP in the QLL-MIA range from 29.1 ng L−1 for ciprofloxacin (CIP) to 8000.0 ng L−1 (8.0 μg L−1) for trovafloxacin (TRO), 20.0 ng L−1 for ceftiofur (CEF) to 3409.1 ng L−1 (3.4 μg L−1) for cephalexin (CEL), 8.6 ng L−1 for sulfadimethoxine (SDM) to 328.2 ng L−1 (0.3 μg L−1) for sulfadiazine (SDZ), and 6.0 ng L−1 for CAP. The recoveries ranged from 80.6% to 105.5% at a fortified concentration of LOD and 2 LOD, with a coefficient of variation <15%. Analysis of field milk samples by the combination of QLL-MIA and the automated pretreatment system (the developed QLL-MIA) was in accordance with that of liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). The above results demonstrated that the developed QLL-MIA could simultaneously screen trace amounts of FQs, β-lactams, SAs, and CAP in field samples, suitable for high-throughput screening of low-molecular weight contaminants.


 Please wait while we load your content...
Please wait while we load your content...