Validation of HPLC and UHPLC methods for the simultaneous quantification of ferulic acid and nicotinamide in the presence of their degradation products†
Abstract
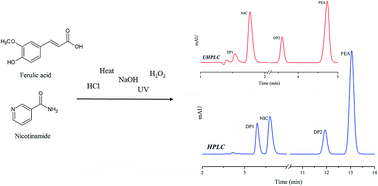
Ferulic acid (FEA) and nicotinamide (NIC) are molecules with antioxidant and anti-inflammatory properties employed in the pharmaceutical field, with special reference to their skin protection and recovery activities. In this study, new HPLC and UHPLC RP methods were developed and validated for quantification of FEA and NIC in the presence of their degradation products. The method developed in HPLC was initially transferred to UHPLC and six different stationary phases were tested, in which a core shell column was chosen. In both methods, diode-array detectors and C18 columns were used. The mobile phase in both methods consisted of phosphate buffer 20 mM and methanol, the solvent proportion being 70 : 30 and 75 : 25 for HPLC and UHPLC, respectively. The oven temperature was set at 40 °C, while flow rates were 0.4 mL min−1 and 1.0 mL min−1 for UHPLC and HPLC, respectively. The wavelength was 268 nm for HPLC and 262 nm for UHPLC. The FEA and NIC forced degradation studies resulted in photo and basic degradation products, nicotinic acid being the degradation product of NIC under basic conditions and the cis isomer of FEA acid its photo-degradation product. For both methodologies, good values of chromatographic parameters were obtained. The accuracy and precision values were close to 100% and 2%, respectively, while R2 > 0.998, for both molecules in HPLC and UHPLC. The LOD, LOQ and robustness evaluation were satisfactory. In conclusion, both developed methodologies were validated and are available to be applied to the analyses of FEA and NIC raw materials.


 Please wait while we load your content...
Please wait while we load your content...