Development of a convenient method for the determination of dimethyl sulfoxide in lyophilised pharmaceuticals by static headspace gas chromatography-mass spectrometry
Abstract
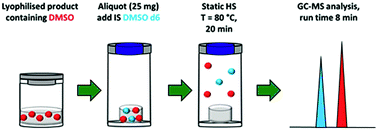
According to the European Pharmacopoeia and the United States Pharmacopeia dimethyl sulfoxide (DMSO) is a class 3 solvent and it is used in the pharmaceutical industry amongst others as an additive for the lyophilisation process. DMSO has a very low vapour pressure and is therefore difficult to remove during freeze drying, as a result a significant amount of the applied DMSO remains in the dried product. For toxicological reasons, as well as for transport and storage, it is important to know about the amount of residual solvents in lyophilised products. Hence, there is a need for a validated method for the determination of the content of residual DMSO, but none of the pharmacopeias provide a concise method for this determination. We have worked out a convenient method for the determination of DMSO in lyophilised pharmaceuticals by static headspace gas chromatography coupled with mass spectrometry which requires only 25 mg of sample. The sample is placed in a 20 mL headspace vial and incubated for 20 min with shaking at 350 rpm at 80 °C before analysis. The method is linear in the range of 0.05–10.00 mg DMSO, the LOQ was set as the lowest point of the calibration curve, and the mean recovery was 108.5%. This fully validated method was applied for the analysis of lyophilised pharmaceuticals with different starting DMSO amounts (2.75–22.00 mg).


 Please wait while we load your content...
Please wait while we load your content...