Microfluidic co-culture of liver tumor spheroids with stellate cells for the investigation of drug resistance and intercellular interactions
Abstract
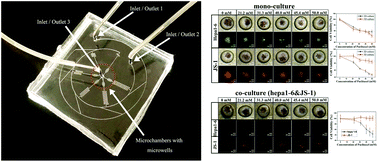
Hepatic stellate cells (HSCs), a major component of the tumor microenvironment in liver cancer, play important roles in cancer progression as well as drug resistance. Here, we presented a microchannel plate-based co-culture model that integrated Hepa1–6 tumor spheroids with JS-1 stellate cells in three-dimensional (3D) concave microwells to mimic the in vivo tumor microenvironment by recapitulating epithelial–mesenchymal transition and chemoresistance. The expression of epithelial–mesenchymal transition (EMT)-related markers and factors was analyzed using immunofluorescent staining and the changes in viability following exposure to different concentrations of paclitaxel were measured. Cell spheroids formed 3D tumor spheroids within 3 days. Culture conditions were optimized for Hepa1–6 cells and JS-1 cells, and their appropriate interactions were confirmed by reciprocal activation. JS-1 under co-culture showed a change in cellular morphology and an increased expression of α-SMA. The expression of EMT-related markers, such as vimentin and TGF-β1, was higher in the co-cultured Hepa1–6 spheroids compared to that in mono-cultured spheroids. Following paclitaxel exposure, JS-1 cells showed significant changes in survival under both mono- and co-culture conditions, while Hepa1–6 presented negligible changes. The proposed microfluidic platform makes it possible to observe the positioned three-dimensional cell spheroids, which would be extensively used not only for well-organized spheroid creation, but also for better quantitative and qualitative understanding of the cell–cell interaction effect.
- This article is part of the themed collection: Analyst Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...