Structural analysis of peptides modified with organo-iridium complexes, opportunities from multi-mode fragmentation†
Abstract
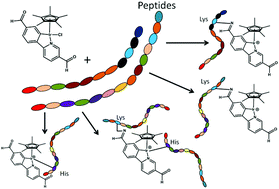
The most widely used anticancer drugs are platinum complexes, but complexes of other transition metals also show promise and may widen the spectrum of activity, reduce side-effects, and overcome resistance. The latter include organo-iridium(III) ‘piano-stool’ complexes. To understand their mechanism of action, it is important to discover how they bind to biomolecules and how binding is affected by functionalisation of the ligands bound to iridium. We have characterised, by MS and MS/MS techniques, unusual adducts from reactions between 3 novel iridium(III) anti-cancer complexes each possessing reactive sites both at the metal (coordination by substitution of a labile chlorido ligand) and on the ligand (covalent bond formation involving imine formation by one or two aldehyde functions). Peptide modification by the metal complex had a drastic effect on both Collisonally Activated Dissociation (CAD) and Electron Capture Dissociation (ECD) MS/MS behaviour, tuning requirements, and fragmentation channels. CAD MS/MS was effective only when studying the covalent condensation products. ECD MS/MS, although hindered by electron-quenching at the Iridium complex site, was suitable for studying many of the species observed, locating the modification sites, and often identifying them to within a single amino acid residue.


 Please wait while we load your content...
Please wait while we load your content...