Ultrasensitive colorimetric detection of Hg2+ ions based on enhanced catalytic performance of gold amalgam dispersed in channels of rose petals
Abstract
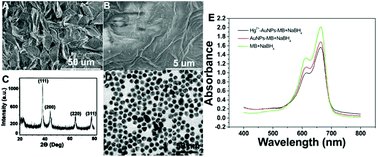
We herein present a simple, low-cost, and ultrasensitive colorimetric sensing strategy for the detection of mercury ions (Hg2+) that takes advantage of the natural pore structure in rose petals to encapsulate gold nanoparticles (AuNPs). AuNPs easily aggregate in high salt concentrations, which restrict their application, for example, as a catalyst in real sample analysis. Rose petals offer a three-dimensional (3D) space for more efficient dispersability of AuNPs in their extensive network of channels, which will increase the specific surface area, thereby enhancing the catalytic capability of AuNPs. The presence of Hg2+ triggers the formation of gold amalgam, which results in remarkable enhancement of the catalytic ability of citrate-capped AuNPs. On the basis of this effect, a colorimetric signal amplification is achieved via the use of gold amalgam to catalyze the reaction of methylene blue (MB) and sodium borohydride. A limit of detection (LOD) as low as 10 fM can be obtained by naked eye observation. Our sensor has promising potential for detection and quantitation of Hg2+ in environmental samples.


 Please wait while we load your content...
Please wait while we load your content...