Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin†
Abstract
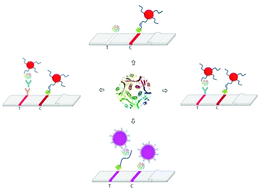
Aptamers are envisioned to serve as powerful synthetic substitutes to antibodies in a variety of bioanalytical assay formats. However, lateral flow assays (LFAs) remain dominated by antibody-based strategies. In this study, a LFA for the detection of cholera toxin as a model analyte is developed and optimized using a synthetic aptamer and a naturally occurring receptor as biorecognition elements and directly compared with solely aptamer and aptamer and antibody-based alternative approaches. The aptamer (CT916) recently selected by our group, GM1 receptors and an anti-cholera toxin antibody were evaluated. Relying solely on molecules that can easily be synthesized while aiming for high sensitivity, we applied a novel combination of capture aptamer and GM1 cell receptor-labeled liposomes for cholera toxin detection, achieving a limit of detection (LOD) of 2 ng ml−1 (3σ)/10 ng ml−1 (visual) in ∼15 min. To put our novel aptasensor into perspective, we developed a competitive lateral flow assay, exploiting the competition of cholera toxin in solution with immobilized cholera toxin for binding of aptamer-coated gold nanoparticles (AuNPs) (LOD = 51 ng ml−1 (3σ)/100 ng ml−1 (visual), assay time ∼10 min). As dual simultaneously binding aptamers were not available, we designed aptamer antibody pair-based lateral flow assays using aptamer-coated AuNPs which yielded a LOD of 5 ng ml−1 (by the 3σ rule)/10 ng ml−1 (visual) in a 10 min assay and an even better LOD of 0.6 ng ml−1 (3σ)/1 ng ml−1 (visual), with an ∼20 min total assay time. All set-ups are highly specific and provide an excellent alternative for cholera toxin detection in places where professional knowledge and sophisticated equipment are not readily available and cost efficient, simple, and rapid tests are needed, while the combination of GM1 cell receptor-labeled liposomes and aptamers is clearly the most promising.


 Please wait while we load your content...
Please wait while we load your content...